What is Mass Spectrometry? Unveiling the Secrets of Molecules
In the realm of analytical chemistry and scientific discovery, mass spectrometry stands as a formidable tool that has revolutionized our understanding of molecules and their intricate properties. From unraveling the composition of compounds to identifying trace elements, this technique has left an indelible mark on fields as diverse as chemistry, biology, forensics, and environmental science. In this comprehensive article, we embark on a journey through the world of mass spectrometry, exploring its principles, applications, and its profound impact on unraveling the mysteries of matter.
Understanding Mass Spectrometry:
At its core, mass spectrometry is a technique used to determine the mass and composition of molecules. It achieves this by ionizing molecules, separating them based on their mass-to-charge ratio, and detecting the resulting ions.
Principles of Mass Spectrometry:
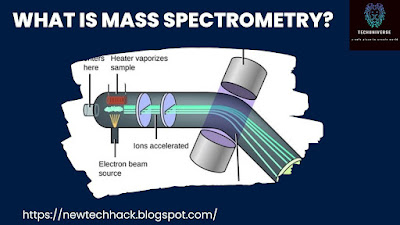
Ionization: The sample is ionized, meaning atoms or molecules are converted into charged ions by various methods, such as electron impact, laser ablation, or electrospray.
Separation: The ions are then subjected to an electric or magnetic field, causing them to move at different speeds based on their mass-to-charge ratio.
Detection: The separated ions are detected, and their relative abundance is recorded as a mass spectrum—a graph showing the intensity of ions at various mass-to-charge ratios.
Applications of Mass Spectrometry:
Compound Identification: Mass spectrometry is used to identify unknown compounds in complex mixtures. It's a crucial tool in drug discovery, environmental analysis, and forensics.
Proteomics and Genomics: Mass spectrometry plays a pivotal role in analyzing proteins and DNA, aiding in understanding biological processes and diseases.
Quantitative Analysis: The technique allows precise quantification of compounds in a sample, even at very low concentrations.
Isotope Ratio Analysis: Mass spectrometry can determine the ratios of isotopes in a sample, helping in fields like geology, archaeology, and ecology.
Drug Metabolism Studies: Mass spectrometry assists in studying how drugs are metabolized in the body, influencing drug design and safety assessments.
Environmental Monitoring: It's used to detect pollutants, trace elements, and contaminants in air, water, and soil samples.
Advancements and Challenges:
High-Resolution Mass Spectrometry: Advancements in instrumentation allow for higher accuracy in determining the mass of ions.
Tandem Mass Spectrometry: This technique involves multiple stages of mass analysis, providing more detailed structural information.
Data Analysis: Handling the large volumes of data generated by mass spectrometry requires sophisticated data analysis tools.
Conclusion:
Mass spectrometry has transcended the realm of analytical chemistry to become a cornerstone of modern scientific exploration. Its ability to unravel the mysteries of molecules—be they drugs, proteins, pollutants, or ancient artifacts—has opened doors to deeper insights and new avenues of research. As technology continues to advance, mass spectrometry will remain a steadfast companion in our quest to understand the intricacies of matter, ushering in a world where molecules yield their secrets to the discerning gaze of scientific inquiry.





No comments:
Post a Comment